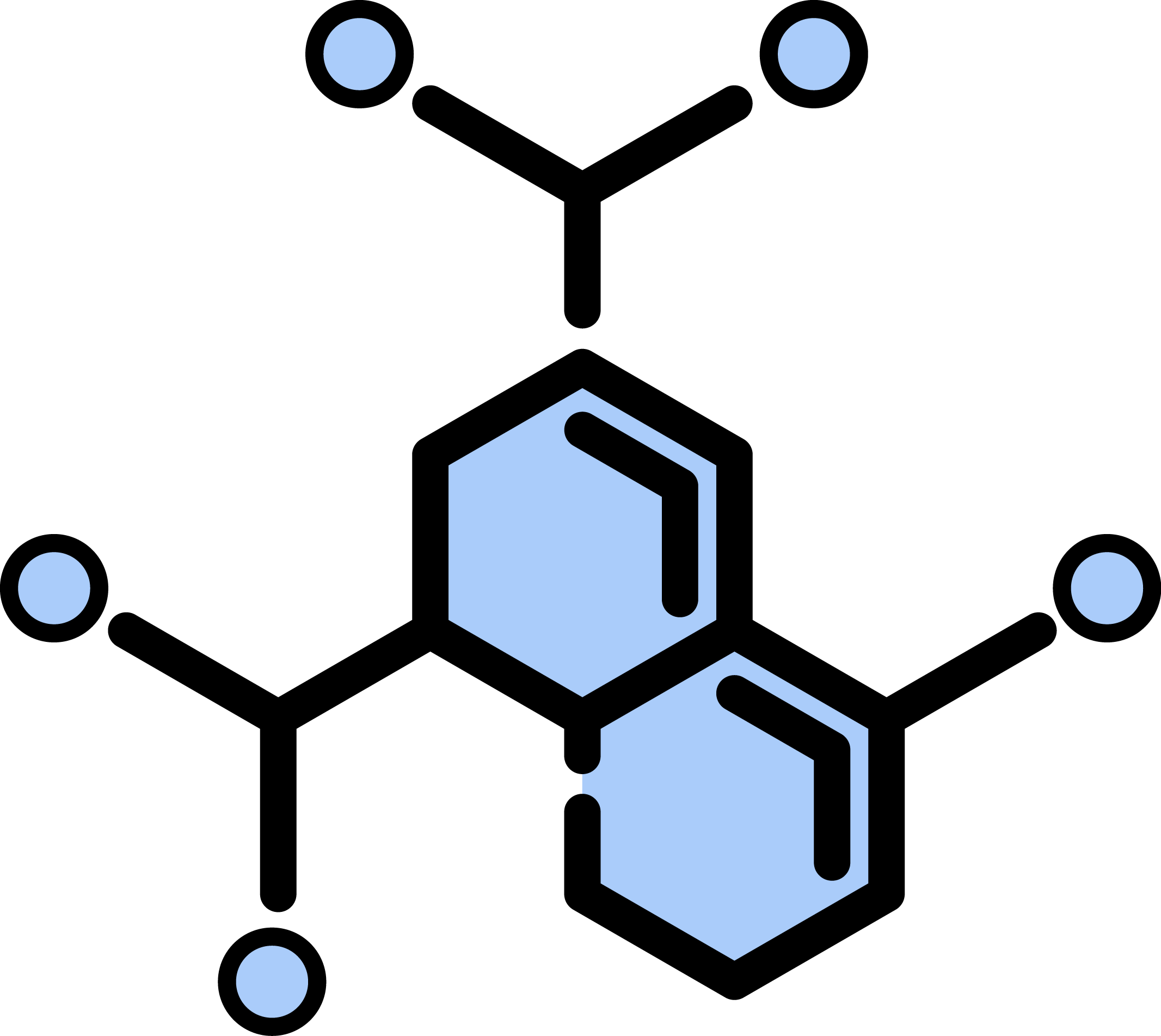
Phosphosite: 253 in O43524
| UniProt ID | O43524 |
|---|---|
| Protein Name | Forkhead box protein O3 (AF6q21 protein) (Forkhead in rhabdomyosarcoma-like 1) |
| Gene Name | FOXO3 |
| Position | 253 |
| SequenceWindow | APRRRAVSMDNSNKY |
| Function Score? | 0.999 |
| Disorder score? | 0.694 Disordered |
| Protein-Targeted Drug | SYRINGARESINOL; RESVERATROL . . . more
|
| Protein Subcellular Localization | Cytoplasm, cytosol {ECO:0000269|PubMed:10102273, ECO:0000269|PubMed:15084260, ECO:0000269|PubMed:16751106, ECO:0000269|PubMed:17711846, ECO:0000269|PubMed:21329882, ECO:0000269|PubMed:22313691, ECO:0000269|PubMed:22761832, ECO:0000269|PubMed:23283301}. Nucleus {ECO:0000269|PubMed:10102273, ECO:0000269|PubMed:15084260, ECO:0000269|PubMed:16751106, ECO:0000269|PubMed:17711846, ECO:0000269|PubMed:21329882, ECO:0000269|PubMed:22313691, ECO:0000269|PubMed:22761832, ECO:0000269|PubMed:23283301, ECO:0000269|PubMed:29445193}. Mitochondrion matrix {ECO:0000269|PubMed:23283301, ECO:0000269|PubMed:29445193}. Mitochondrion outer membrane {ECO:0000269|PubMed:29445193}; Peripheral membrane protein {ECO:0000269|PubMed:29445193}; Cytoplasmic side {ECO:0000269|PubMed:29445193}. Note=Retention in the cytoplasm contributes to its inactivation (PubMed:10102273, PubMed:15084260, PubMed:16751106). Translocates to the nucleus upon oxidative stress and in the absence of survival factors (PubMed:10102273, PubMed:16751106). Translocates from the cytosol to the nucleus following dephosphorylation in response to autophagy-inducing stimuli (By similarity). Translocates in a AMPK-dependent manner into the mitochondrion in response to metabolic stress (PubMed:23283301, PubMed:29445193). Serum deprivation increases localization to the nucleus, leading to activate expression of SOX9 and subsequent chondrogenesis (By similarity). {ECO:0000250|UniProtKB:Q9WVH4, ECO:0000269|PubMed:10102273, ECO:0000269|PubMed:15084260, ECO:0000269|PubMed:16751106, ECO:0000269|PubMed:23283301, ECO:0000269|PubMed:29445193}. . . . more
|
| Protein Function | Transcriptional activator that recognizes and binds to the DNA sequence 5'-[AG]TAAA[TC]A-3' and regulates different processes, such as apoptosis and autophagy (PubMed:10102273, PubMed:16751106, PubMed:21329882, PubMed:30513302). Acts as a positive regulator of autophagy in skeletal muscle: in starved cells, enters the nucleus following dephosphorylation and binds the promoters of autophagy genes, such as GABARAP1L, MAP1LC3B and ATG12, thereby activating their expression, resulting in proteolysis of skeletal muscle proteins (By similarity). Triggers apoptosis in the absence of survival factors, including neuronal cell death upon oxidative stress (PubMed:10102273, PubMed:16751106). Participates in post-transcriptional regulation of MYC: following phosphorylation by MAPKAPK5, promotes induction of miR-34b and miR-34c expression, 2 post-transcriptional regulators of MYC that bind to the 3'UTR of MYC transcript and prevent its translation (PubMed:21329882). In response to metabolic stress, translocates into the mitochondria where it promotes mtDNA transcription (PubMed:23283301). In response to metabolic stress, translocates into the mitochondria where it promotes mtDNA transcription. Also acts as a key regulator of chondrogenic commitment of skeletal progenitor cells in response to lipid availability: when lipids levels are low, translocates to the nucleus and promotes expression of SOX9, which induces chondrogenic commitment and suppresses fatty acid oxidation (By similarity). Also acts as a key regulator of regulatory T-cells (Treg) differentiation by activating expression of FOXP3 (PubMed:30513302). {ECO:0000250|UniProtKB:Q9WVH4, ECO:0000269|PubMed:10102273, ECO:0000269|PubMed:16751106, ECO:0000269|PubMed:21329882, ECO:0000269|PubMed:23283301, ECO:0000269|PubMed:30513302}. . . . more
|
| Functional domains |
Distribution of Tumor Phosphorylation Levels (corrected without protein) in Pan-Cancer
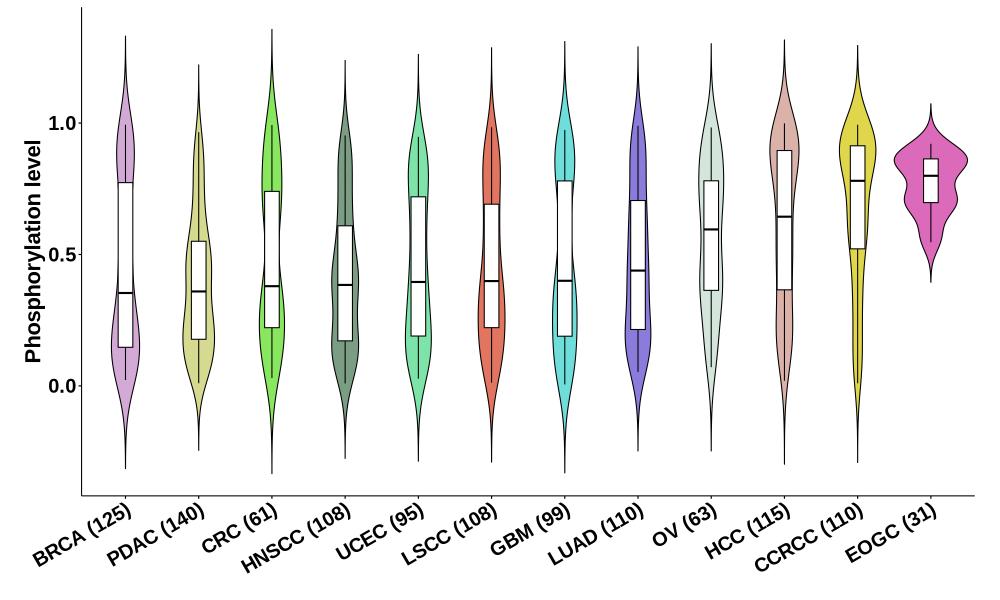
Distribution of Tumor Phosphorylation Levels (corrected with protein) in Pan-Cancer

Distribution of Normal Phosphorylation Levels (corrected without protein) in Pan-Cancer
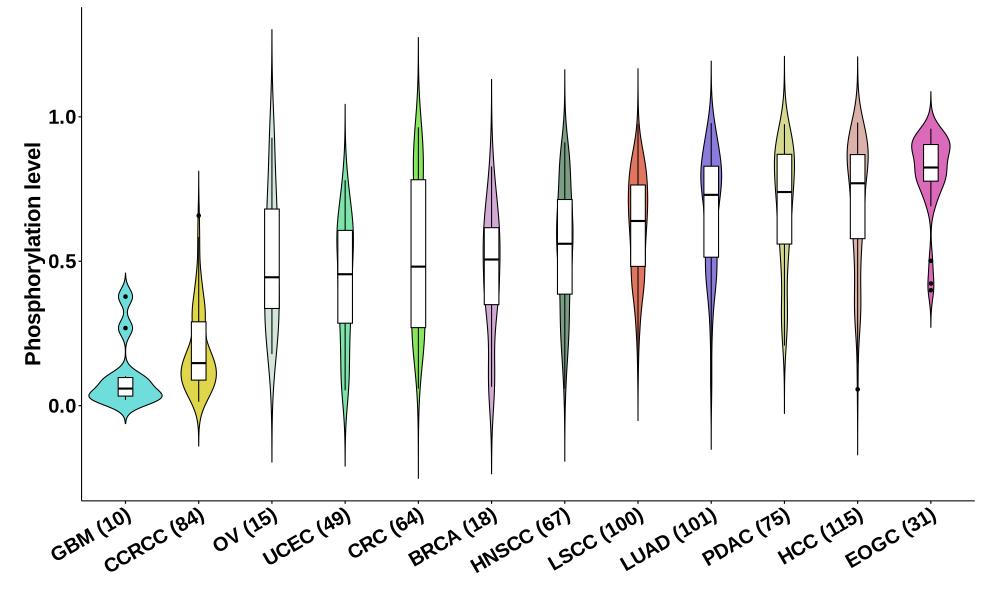
Distribution of Normal Phosphorylation Levels (corrected with protein) in Pan-Cancer

Copyright © Jing Li's group, SJTU. All rights reserved.